Cytokine receptor cluster size impacts its endocytosis and signaling
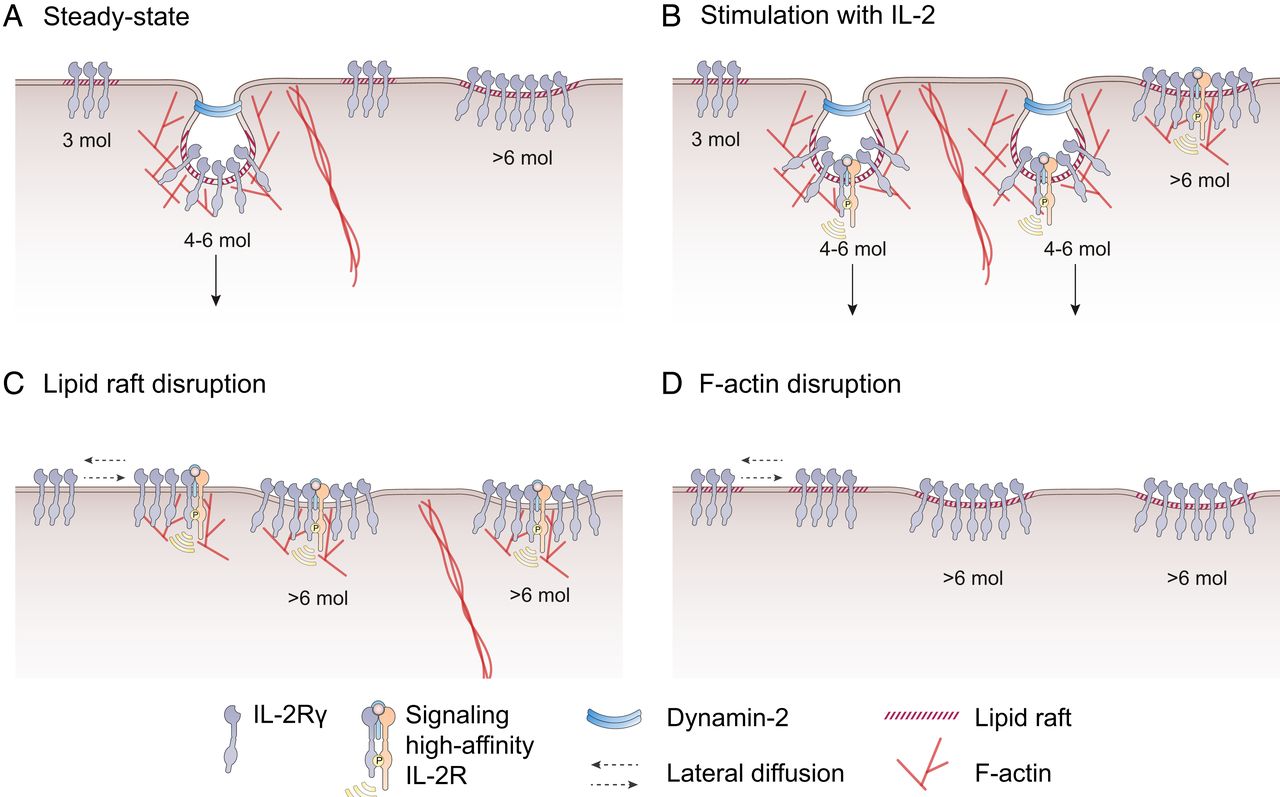
Model for IL-2R clustering
A collaborative work between teams of Prof. Grassart and Prof. Sansonetti from the Center for Microbes, Development and Health at the Institut Pasteur of Shanghai-Chinese Academy of Science and teams of Dr. Sauvonnet and Dr. Olivo-Marin from the Institut Pasteur Paris (France) and team from Sorbonne University (France) recently revealed new insights into the mechanism of endocytosis and signaling of the receptor for interleukin-2, a key player in immune response. This work has been published in Proceeding of the National Academy of Sciences of the United States of America (PNAS) on September 14 2021.
Interleukin-2 (IL-2) is a well-known cytokine playing a central role in immunity and being implicated in auto-immune diseases. Upon cytokine binding to its specific receptors (IL-2R) at the plasma membrane, a signaling cascade is transduced to stimulate lymphocytes proliferation. The internalization of receptors, a process also called endocytosis, has been previously demonstrated as an important mechanism for terminating this signaling transduction. However, the mechanism by which IL-2R initiate its endocytosis remained poorly understood so far.
Here, by applying genome-engineering, quantitative single molecule imaging and mathematical modeling, authors revealed the native dynamics of Il-2R during endocytosis at the surface of lymphocytes. In particular they discovered an original stoichiometric organization of Il-2R into clusters regulating endocytosis and signaling. In contrast to classical endocytosis model of many receptors’ uptake, mediated through clathrin pit, the internalization of Il-2R receptors use an unconventional pathway. Authors found that IL-2R following endocytic activity were engulfed into large clusters, not- coated by clathrin molecules and composed of an average of 6 cytokine receptors. The authors also determined two other specific organizations of IL-2R at the plasma membrane. First, they identified that receptors could be arranged into big platforms that are apparently less efficient for internalization. Second, they quantified that the majority of IL-2 receptors at the plasma membrane exist also into smaller clusters, composed of a maximum of 3 molecules, which they identified as diffusive or secreted receptors. Finally, authors demonstrated that cholesterol and branched actin cytoskeleton control this specific receptor clustering organization. Indeed, perturbation of these factors led to a stochiometric disorganization of IL-2R clustering which eventually led to profoundly impact IL-2R endocytosis and signaling.
Overall, this work provides important insights into our understanding of the mechanism initiating the clathrin-independent endocytosis followed by IL-2R. Because protein clustering and oligomerization are common process into the cell, this study opens new avenues for better understanding many other cellular events. Finally, this work provides new evidence for the critical biophysical aspects in controlling biochemical properties and cellular homeostasis.