New mechanism for cGAMP cell-to-cell transmission and STING activation during viral infection
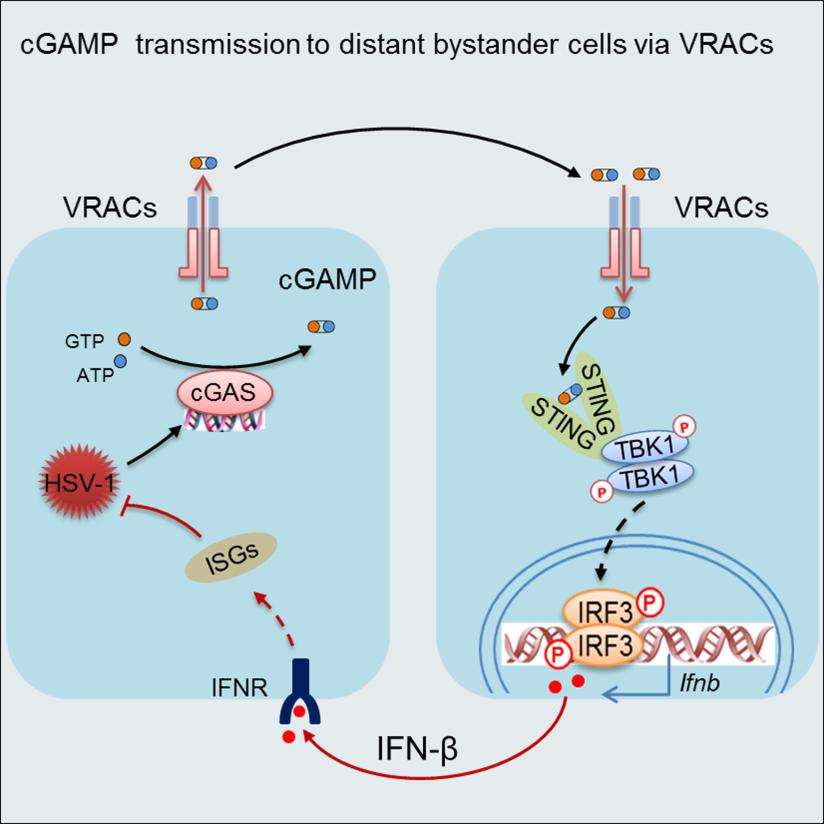
VRAC-mediated cGAMP transmission leads to the activation of STING signaling in bystander cells and the induction of reinforcing IFN response to curb viral propagation in virus-infected cells.
On April 10, the Cell Press journal IMMUNITY online published the research article “Transfer of cGAMP into Bystander Cells via LRRC8 Volume-Regulated Anion Channels (VRACs) Augments STING-Mediated Interferon Responses and Anti-viral Immunity”. This study demonstrated that the cell membrane anion channel LRRC8/VRAC is a cGAMP transporter that can transmit cGAMP from virus-infected cells to non-infected cells to activate STING signaling and interferon responses. This finding revealed a unique way of cooperation between infected cells and non-infected cells during viral infections, thus providing valuable insights into the development of vaccines and therapeutic intervention of infectious disease and cancer.
Dr. Hui Xiao’s group has a long-standing interest in the identification of novel components of the so-called pattern-recognition receptor signaling pathways and the elucidation of immune mechanisms involved in antiviral defense. Through a cell-based screening platform previously established by themselves, they screened for a library of small chemical compounds targeting anion channels. They then made an unexpected observation that the anion channel LRRC8/VRAC, which is best known for its crucial role in the cell-swelling response, had a pivotal role in the host defense against the DNA virus herpes virus HSV-1. Whereas HSV-1 infection triggers the activation of the cytosolic DNA sensor cGAS, which then synthesizes the second message cGAMP to activate STING for the IFN-I response, VRAC does not seem to affect cGAS activation or cGAMP production in HSV-1 infected cells. Instead, the whole-cell patch-clamp electrophysiological analyses and liquid chromatography-tandem mass spectrometry assays collectively established LRRC8/VRAC as a cGAMP transporter. By conducting both cGAMP efflux and influx along the concentration gradient, VRAC promoted the activation of STING signaling and the induction of IFN-I responses in uninfected bystander cells. Whereas the hypotonic condition being the most potent stimulus for VRAC activation, the researchers further identified proinflammatory cytokines TNF and IL-1 as potential activators for VRAC. Presumably, during viral infection, VRAC can be opened by proinflammatory cytokines to transport cGAMP from infected cells to the distant bystander cells, wherein eliciting reinforcing IFN response. In support of this hypothesis, the genetic ablation of VRAC in mice led to diminished IFN responses but heightened viral propagation during HSV-1 infection. These results demonstrate that in addition to its well-established role in transport chloride and organic compounds such as aspartate, glutamate and cisplatin, VRAC also has a crucial role in conducting cGAMP transmission for host defense. Hence, these results implicate an unexpected contribution of VRAC to the antiviral IFN response from bystander cells. Since LRRC8/VRAC can transport cGAMP from both virus-infected and cancerous cells, this study presents a new avenue for the development of vaccine and immunotherapy for infectious diseases and cancers.
This study was led by Dr. Hui Xiao’s group at Institut Pasteur of Shanghai (IPS), in collaboration with Dr. Thomas J. Jentsch’s group at Leibniz Institute of Molecular Pharmacology in Germany (FMP/MDC) and Dr. Zhaozhu Qiu’s group at Johns Hopkins University. Zhou Chun, a Ph.D. student at the University of The Chinese Academy of Sciences, Chen Xia, a Ph.D. student at Lanzhou University/IPS, and Rosa Planells-Cases, a postdoctoral fellow at FMP/MDC, are the co-first authors of this paper, with Drs. Hui Xiao, Thomas J. Jentsch and Zhaozhu Qiu being the corresponding authors.
This study was supported by the Key Development and Research project of China; the National Natural Science Foundation of China; the Chinese Academy of Sciences; the Shanghai Municipal Science and Technology; the European Research Council Advanced Grant, European Union; and the Klingenstein-Simons Fellowship in Neuroscience and the Sloan Research Fellowship, USA.